Electron delocalization and aromaticity in excited states
Bachelor or Master Thesis Project
Type of Project: Theoretical
Location: DIPC, Donostia / San Sebastián
Supervisor at DIPC: Irene Casademont (DIPC), Miquel Torrent (UPV/EHU, DIPC associate), Eduard Matito (DIPC)
ematito@dipc.org
Aromaticity is one of the most fundamental concepts in chemistry, particularly in organic chemistry. It arises from cyclic electron delocalization (or conjugation) in closed systems, leading to characteristic features such as bond-length equalization, energy stabilization, significant magnetic anisotropies, and unusual chemical shifts, among other well-documented effects.
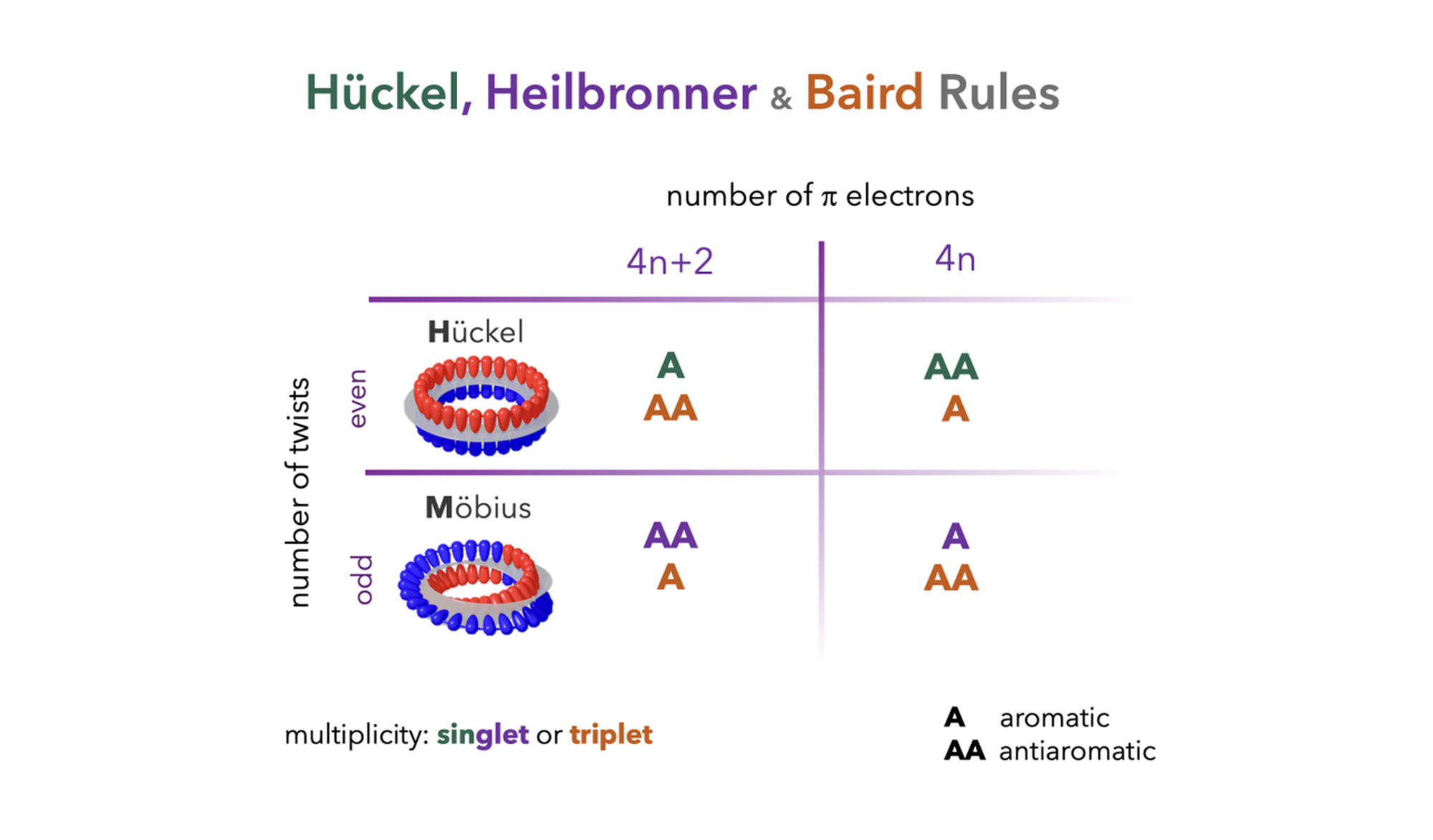
The aromaticity of organic compounds is qualitatively determined by their topology, spin state, and the number of π-electrons, as described by Hückel's, Heilbronner's, and Baird's rules. According to Hückel's rule, a planar π-conjugated system (or one with an even number of half-twists) in the singlet state is aromatic (antiaromatic) if it contains 4N+2 π-electrons and antiaromatic if it contains 4N π-electrons. In contrast, Heilbronner's rule predicts a reversal for planar π-conjugated systems with an odd number of half-twists: a singlet Möbius system is antiaromatic with 4N+2 π-electrons and aromatic with 4N π-electrons. This diversity of aromatic patterns is expanded when triplet states are considered. According to Baird's rule, the aromaticity/antiaromaticity electron-counting rules of the singlet ground state are reversed in the lowest π-π* triplet state, doubling the range of possible aromatic topologies.
In a recent study [1], we conducted a theoretical investigation into the conformational switching among Hückel planar, singly twisted Möbius, and figure-eight conformations of [26]- and [28]-hexaphyrins, focusing on both their ground state and lowest excited triplet state. Notably, we found that while Baird's rule is qualitatively applicable to these compounds, there is a significant exception: the Hückel planar [26]-hexaphyrin in the triplet state exhibits pronounced aromatic character.
For this bachelor/master thesis, the candidate will learn how to quantify aromaticity using computational chemistry tools, focusing on electronic and magnetic descriptors [2-6]. The aromaticity of selected macrocycles will be analyzed in both the ground state and the lowest excited states within a density functional theory (DFT) framework. The ultimate goal is identifying macrocycles that deviate from the aforementioned rules. We are seeking highly motivated candidates with a foundational understanding of quantum chemistry—typically acquired through undergraduate studies in chemistry or physics—and a strong desire to learn the fundamentals of electronic structure theory and density functional theory (DFT).
[1] Casademont-Reig I., Matito E., Torrent-Sucarrat M. (submitted)
[2] Casademont-Reig I., Woller T., Contreras-García J., Alonso M., Torrent-Sucarrat M., Matito E., Phys. Chem. Chem. Phys. 20, 2787 (2018)
[3] Casademont-Reig I., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Molecules 25, 711 (2020)
[4] Casademont-Reig I., Guerrero-Avilés R., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Angew. Chem. Int. Ed. 60, 24080 (2021)
[5] Casademont-Reig I., Soriano-Agueda L., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Angew. Chem. Int. Ed. 61, e202206836 (2022)
[6] Casademont-Reig I., Woller T., García V., Contreras-García J., Tiznado T., Torrent-Sucarrat M., Matito E., Alonso M., Chem. Eur. J. 29, e202202264 (2023)
