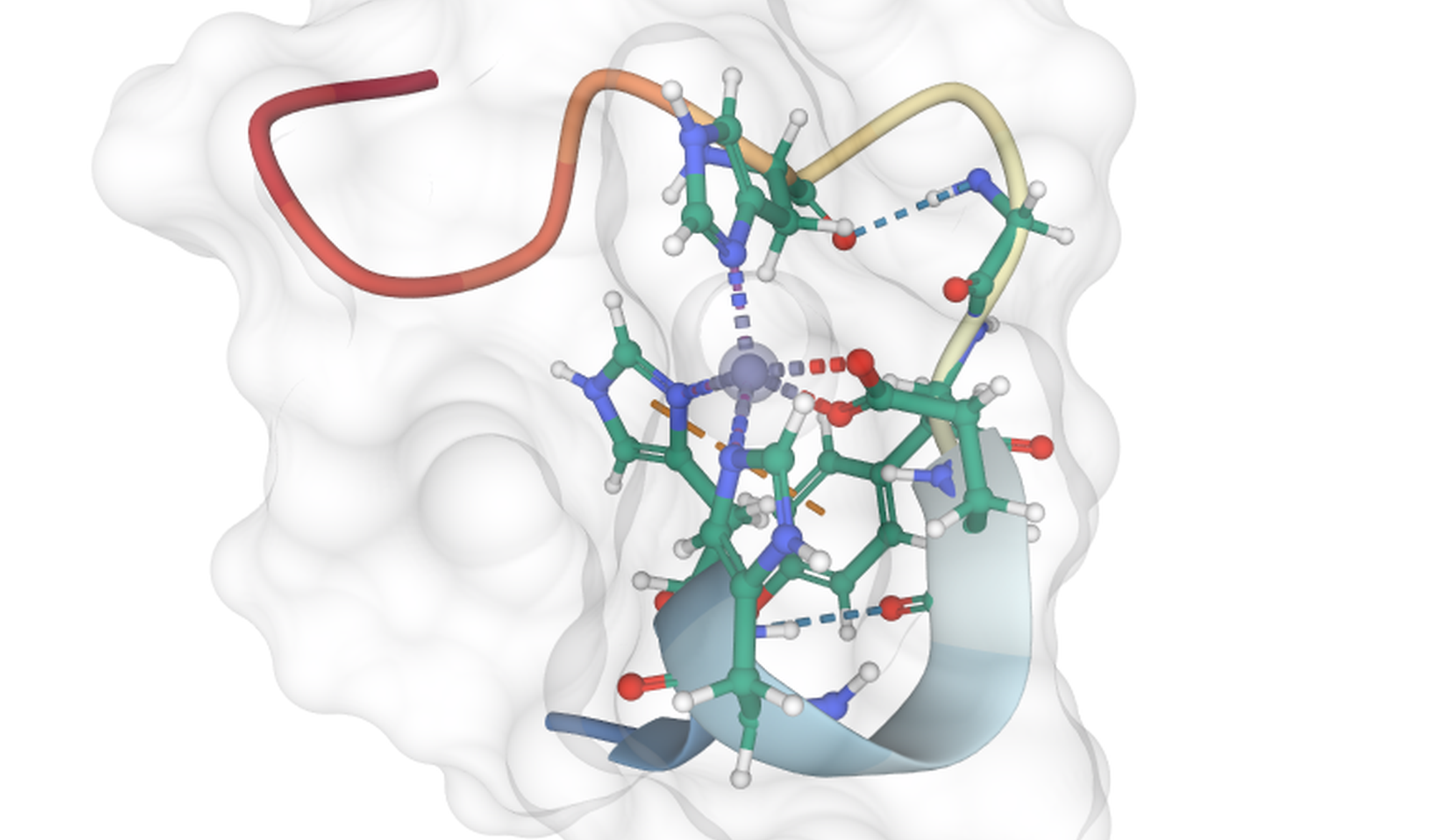
The interaction landscape of amyloid-Zn2+ from molecular dynamics and QM simulations
Internship
Type of Project: Experimental
Location: Donostia
Supervisor:
David de Sancho (webpage)
Txema Mercero
Amyloid fibrils are stable forms of misfolded proteins associated with numerous neurodegenerative diseases. Among these, Alzheimer's disease may be the most prevalent, with over 50 million dementia cases reported by the World Health Organization in 2019. The molecular origin of Alzheimer's is linked to amyloid fibril formation by misfolded Aβ-peptide (Aβ). These fibrils can form aggregates that are stabilized by the presence of Zn2+ cations. Although many possible structures have been reported in the last few years for the Zn2+-Aβ complexes, details about the molecular interactions involved are still lacking.
In this project, we propose a detailed computational study of the different possible interactions between the Zn2+ and the Aβ by means of equilibrium classical molecular dynamics simulations and the Hamiltonian replica exchange method in order to characterize these bound states of Zn2+. Once the possible binding modes have been identified by means of MM, we will employ Quantum Mechanic calculations to understand the interactions occurring between the cation and the peptide.
