Computational study of the effect of pressure on the thermal dimerization of cycloheptatriene
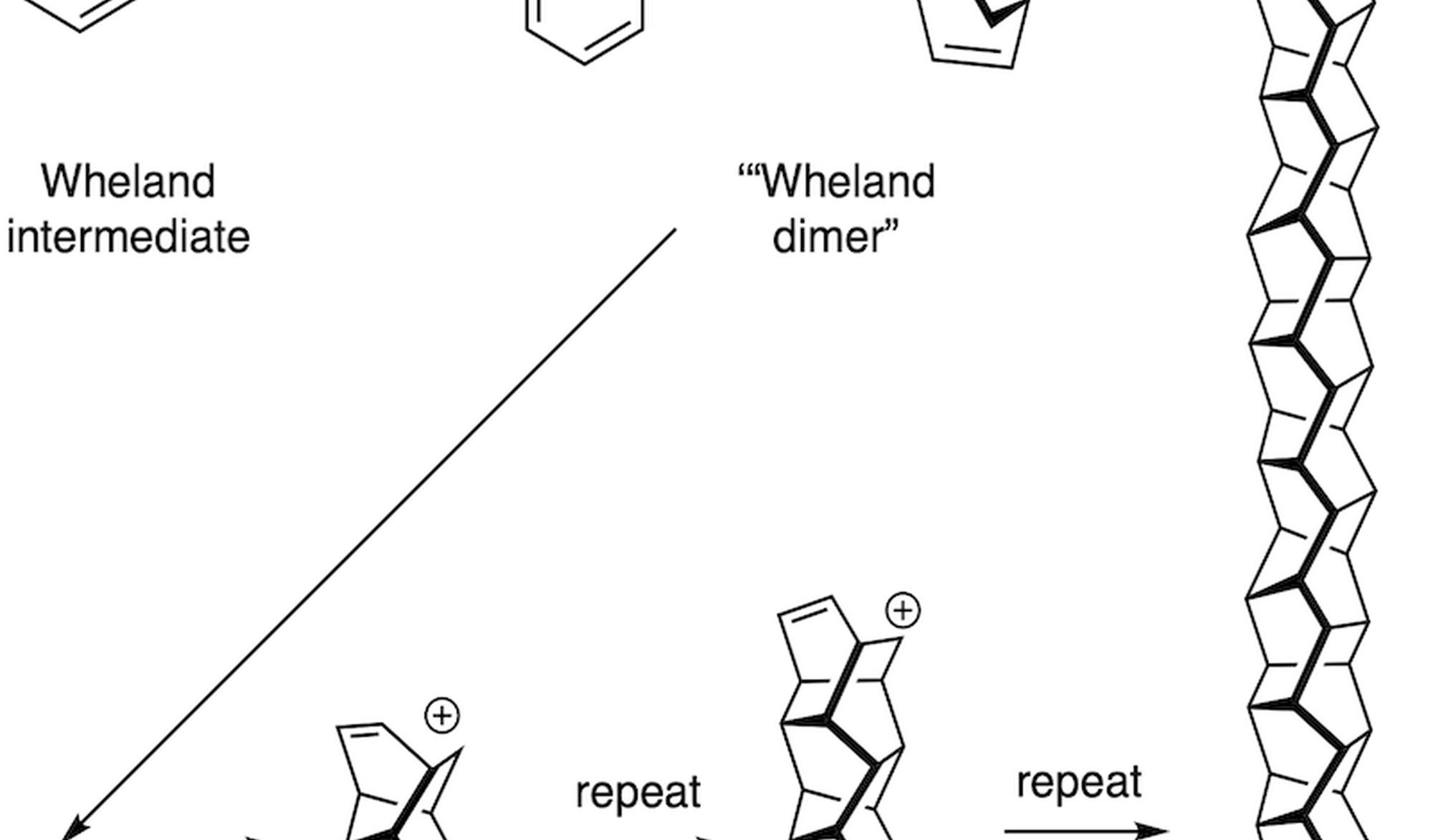
Thermal dimerization is a common reaction of cyclic dienes or trienes. For example, cyclopentadiene readily dimerizes at room temperature to the Diels-Alder dimers. At elevated temperature, 1,3-cyclohexadiene dimerizes to a range of products. Both of these two reactions are well-studied experimentally and computationally, at ambient pressure and high pressure. However, the dimerization of cycloheptatriene has only been studied preliminarily at ambient pressure. There is no report on the effect of pressure on this dimerization. Computational elucidation of this reaction is also missing.
An interesting feature in these dimerizations is the so-called reaction pathway bifurcation, where a transition state is connected directly to a second transition state without an intermediate.
The extreme-pressure polarizable continuum model (XP-PCM) is a recently-developed, quantum chemical method aimed to introduce the effects of the pressure on the calculation of the electronic energy of a molecular system in a dense medium via a Pauli exchange-repulsion interaction between the molecular system and the external medium.
In this project, we will study the free energy surfaces of cycloheptatriene dimerization and the effect of pressure on the reaction profiles. Interesting questions that we attempt to answer include: (a) What is the most favorable reaction mechanism? (b) Does it change at high pressure? (c) Could high pressure alter the potential energy surface to such an extent that the bifurcation disappears?
The student will work on the following tasks:
- use computational chemistry software (for example, Gaussian 16) to optimize the geometries of reactants, transition states, intermediates, and products, and to compute the energies, enthalpies, free energies of the optimized structures
- analyze complex potential energy surfaces involving bifurcation
- use the XP-PCM method to compute high-pressure reaction profiles and activation volumes of competing reaction pathways
The candidate is expected to have a good knowledge of organic chemistry. Experience with molecular modeling/simulation/calculation is preferable but not required.
