Expanding the Scope of Radical Ion Pairs
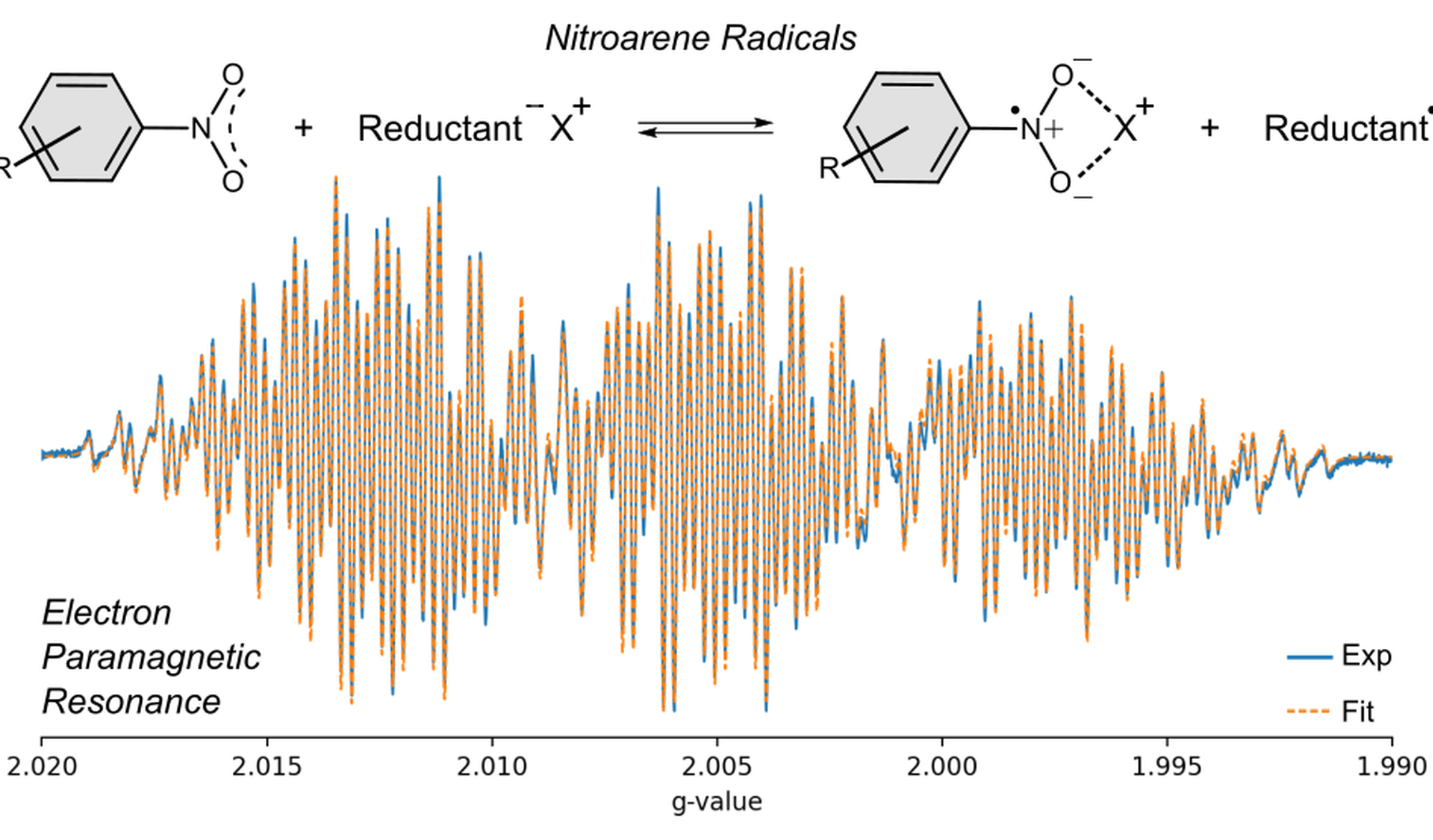
Organic molecular magnetism, i.e., organic molecules capable of hosting unpaired electrons, also called radicals, is a rapidly evolving field that offers exciting applications in diverse areas, ranging from qubits[1] to chemical synthesis[2]. However, the currently available molecular architectures use only a fraction of the available chemical space, inadvertently limiting the full potential this field has to offer. Current efforts in our group aim at remedying this, following the particularly appealing possibility of frustrated Lewis Pairs (FLPs)[3] – we employ a concerted experimental and computational approach to establish and exploit general strategies that introduce radical properties in broader families of organic molecules.
Building on ongoing studies at the group, you will contribute to our most recent research lines by studying the electronic structure of novel radical ion pairs using spectroscopic techniques. In particular, you will:
- Measure the electron paramagnetic resonance (EPR) response of a number of nitroarene radicals.
- Learn how to model the EPR spectra you measure by means of model spin Hamiltonians to obtain structural and electronic information.
- Initiate studies on the regioselective aliphatic C-H activation of these newly formed radical ion pairs.
- Learn how to operate a newly purchased, state-of-the-art EPR spectrometer.
- Learn the programming language Python, as an effective way to treat, organise and visualise data.
- (Optional) Learn how to work on a glovebox for handling air and moisture sensitive chemistry.
The candidate is expected to have a good knowledge of organic chemistry and electronic structure of molecules.
References:
[1] M. Slota et al., Nature, 2018, 557, 691–695.
[2] J. H. Kim et al., Nature, 2021, 595, 677–683.
[3] Z. Lu et al., Nature, 2023, 619, 514–520.
