Unlocking direct C-H activation with radical pairs
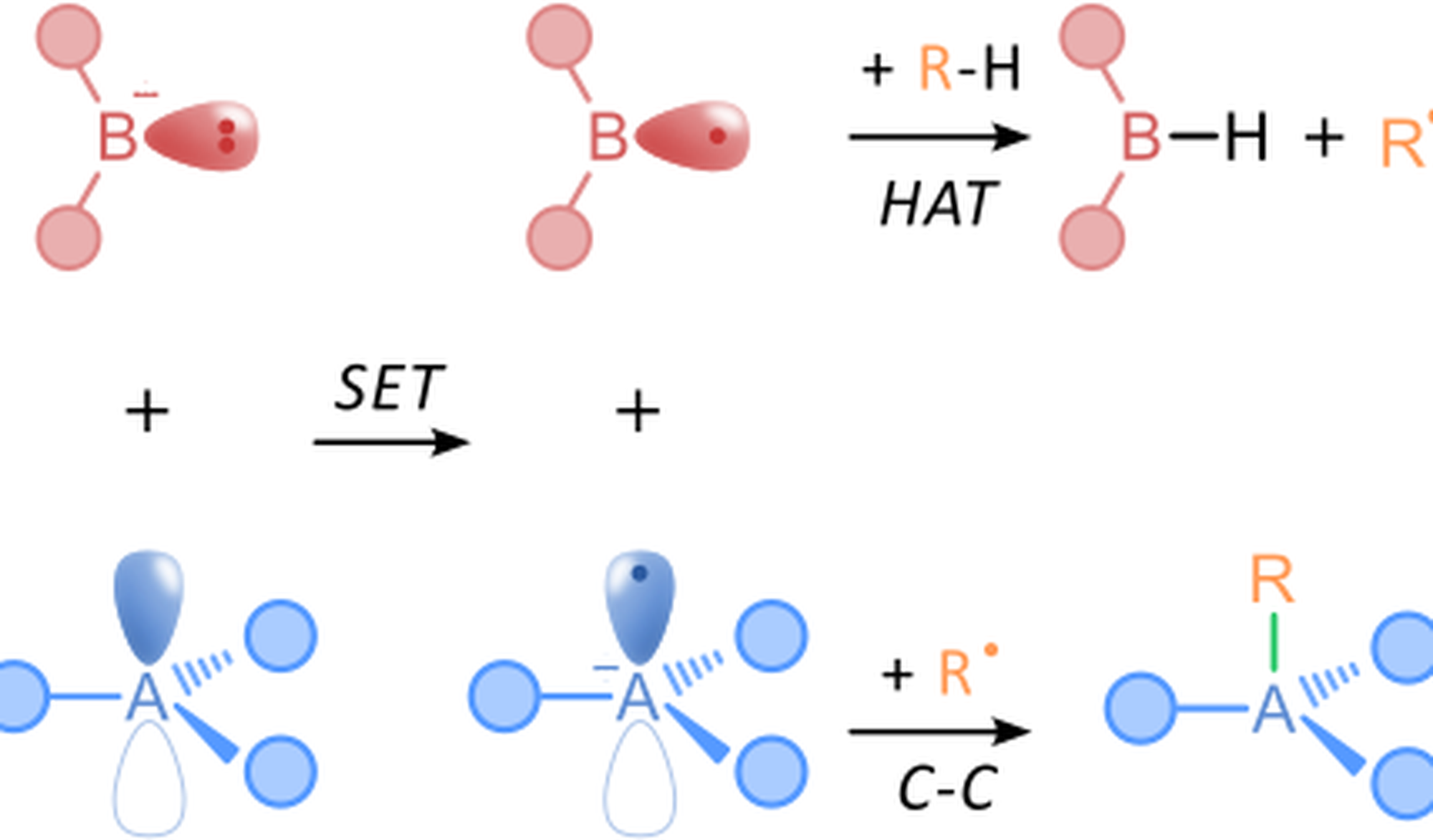
Saturated hydrocarbons represent the most abundant carbon feedstocks, yet their chemical valorisation is severely limited by the intrinsic inertness of aliphatic C–H bonds, which are strong, non-polar, and difficult to differentiate selectively. Conventional functionalization strategies therefore rely on pre-functionalization or harsh oxidative conditions, increasing synthetic inefficiency and waste. Radical-based approaches to direct C–H activation offer a compelling alternative, as open-shell intermediates enable C–H cleavage under comparatively mild conditions while providing access to reactivity patterns inaccessible to closed-shell pathways [1]. By allowing selective hydrogen-atom abstraction and subsequent functionalization without pre-installed handles, radical C–H activation not only improves step and atom economy but also reframes C–H bonds as programmable reaction sites for the late-stage and sustainable upgrading of saturated hydrocarbons.
In our group, we combine experimental and computational strategies to advance radical-based chemistry. In particular, we have recently shown that nitrobenzene induces the formation of potent hydrogen-atom transfer (HAT) radicals [2], opening the door to direct C–H activation under remarkably simple conditions. After HAT from a saturated hydrocarbon, the resulting carbon-centered radicals are primed to engage with the persistent nitrobenzene radical, providing an ideal reaction partner for subsequent radical cross-coupling reactions. This dual reactivity unlocks C–H activation via HAT and C(sp³)–C(sp²) bond formation in one pot, allowing C(sp²) functionalization to occur concomitantly with radical generation. As such, nitrobenzene serves not only as a C–H activation trigger but also as a built-in radical acceptor, unifying activation and functionalization within a single radical manifold.
Building on ongoing studies at the group, you will contribute to our most recent efforts by expanding the scope of C-H activation using radical chemistry. In particular, you will:
• Perform reactions to assess C-H activation and radical cross-coupling reactions. This will involve reaction work-up, product separation by column chromatography and product characterisation by NMR spectroscopy and crystallisation techniques.
• Characterise radical formation by means of electron paramagnetic resonance (EPR) spectroscopy.
• Learn how to work on a glovebox for handling air and moisture sensitive radicals.
• Expand the scope to other nitroaromatic compounds.
• Learn the programming language Python, as an effective way to treat, organise and visualise data.
The candidate is expected to have a good knowledge of organic chemistry and electronic structure of molecules.
References:
[1] Z. Lu et al., Nature, 2023, 619, 514–520.
[2] S. A. Balahoju et al., ACS Omega 2025, 10, 22, 23798–23807
