Proton dynamics in enzymatic processes
Internship
Type of Project: Theory Project
Location: Donostia
Supervisors: Jon Uranga
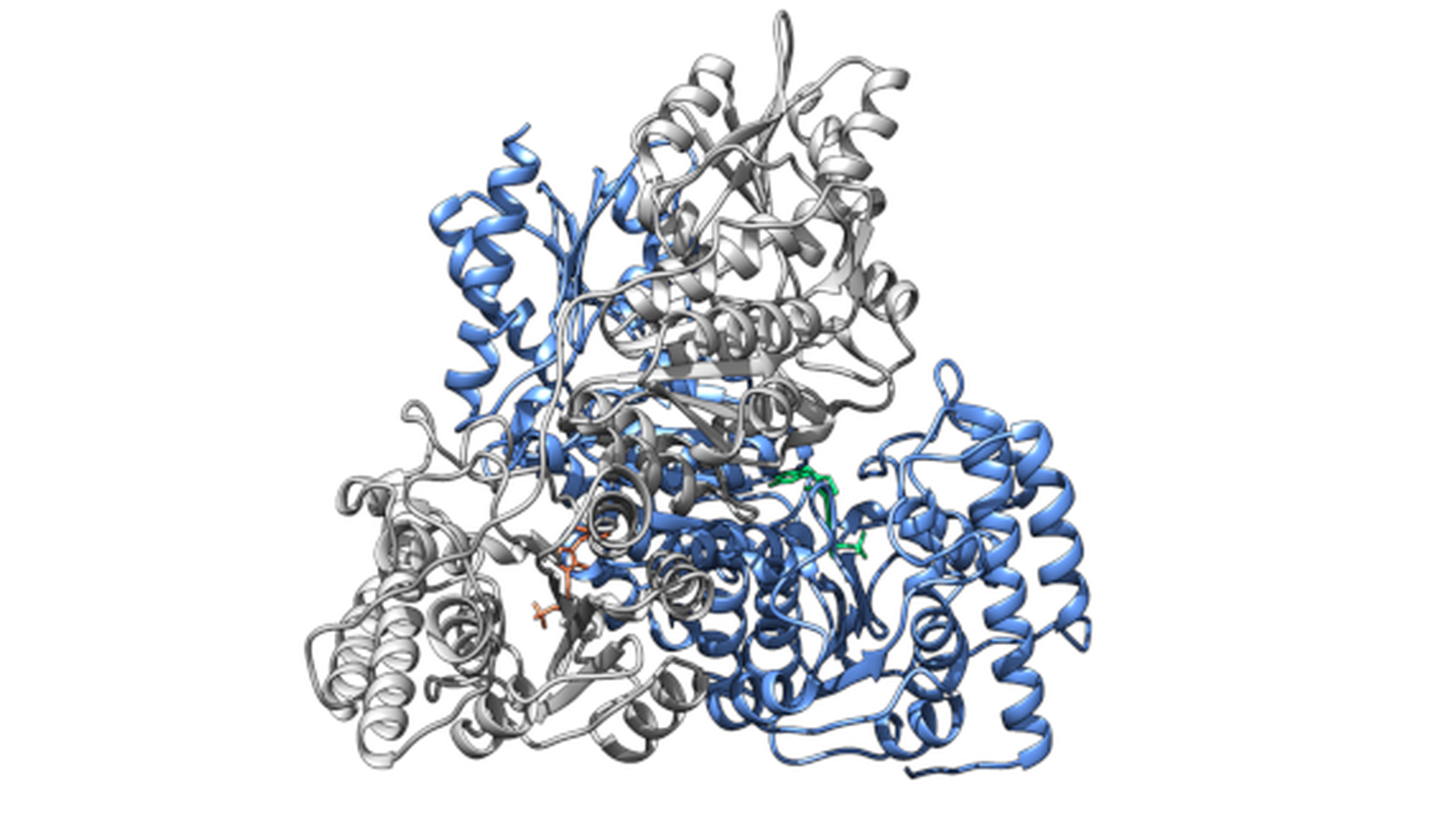
This study examines the critical role that protons play in enzyme function, focusing on how they affect substrate binding, catalysis and regulatory processes, including allostery and cooperativity. Enzymes, which act as biological catalysts, rely on complex molecular interactions and structural dynamics to promote biochemical reactions. Protons play a crucial role in these dynamics, stabilising charged intermediates during catalysis and altering the protonation states of titratable residues to influence enzyme activity.
Using cutting-edge theoretical techniques, the researchers will investigate the mysterious functions of protons in enzyme processes. Using approaches such as Replica Exchange Molecular Dynamics and Quantum Mechanics/Molecular Mechanics (QM/MM) simulations, the study aims to overcome the shortcomings of existing proton characterisation techniques. Determining pKₐ values to adequately represent protonation states is a crucial task in gaining a thorough knowledge of enzyme dynamics at the molecular level. By better understanding how protons contribute to the operation and control of enzymes, this investigation aims to improve our general understanding of enzymatic activities.
