On the validity of the Baird Rule
Internship
Type of Project: Theory Project
Location: Donostia
Supervisors:
Miquel Torrent Sucarrat, Irene Casademont-Reig
Aromaticity is one of the most fundamental concepts in chemistry, particularly in organic chemistry. It arises from cyclic electron delocalization (or conjugation) in closed systems, leading to characteristic features such as bond-length equalization, energy stabilization, significant magnetic anisotropies, and unusual chemical shifts, among other well-documented effects.
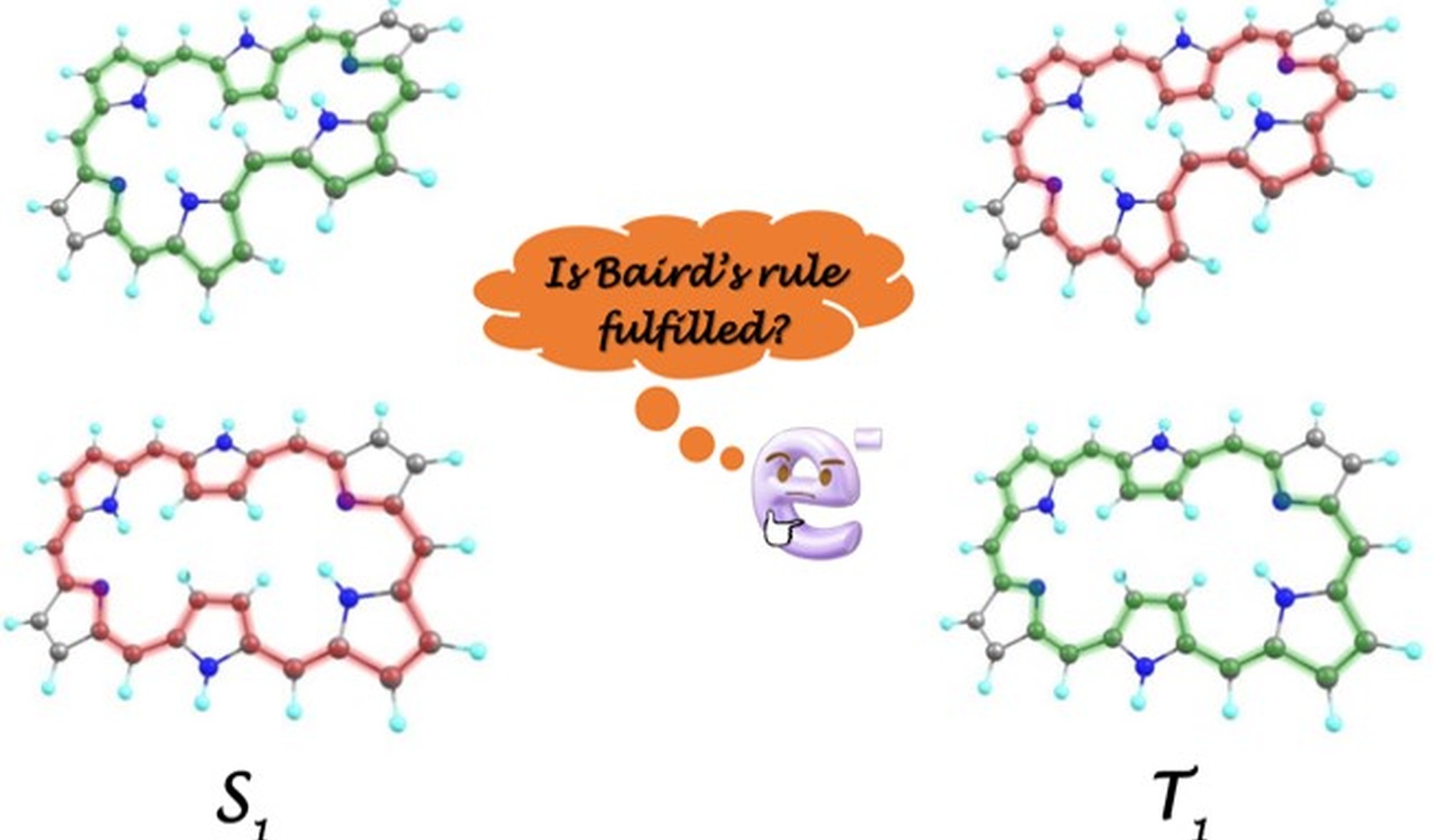
The aromaticity of organic compounds is qualitatively determined by their topology, spin state, and the number of π-electrons, as described by Hückel's, Heilbronner's, and Baird's rules. According to Hückel's rule, a planar π-conjugated system (or one with an even number of half-twists) in the singlet state is aromatic (antiaromatic) if it contains 4N+2 π-electrons and antiaromatic if it contains 4N π-electrons. In contrast, Heilbronner's rule predicts a reversal for planar π-conjugated systems with an odd number of half-twists: a singlet Möbius system is antiaromatic with 4N+2 π-electrons and aromatic with 4N π-electrons. This diversity of aromatic patterns is expanded when triplet states are considered. According to Baird's rule, the aromaticity/antiaromaticity electron-counting rules of the singlet ground state are reversed in the lowest π-π* triplet state, doubling the range of possible aromatic topologies.
In a recent study,[1] we conducted a theoretical investigation into the conformational switching among Hückel planar, singly twisted Möbius, and figure-eight conformations of [26]- and [28]-hexaphyrins, focusing on both their ground state and lowest excited triplet state. Notably, we found that while Baird's rule is qualitatively applicable to these compounds, there is a significant exception: the Hückel planar [26]-hexaphyrin in the triplet state exhibits pronounced aromatic character.
During this internship, the candidate will learn how to quantify aromaticity using computational chemistry tools, focusing on electronic and magnetic descriptors.[2] The aromaticity of selected macrocycles will be analyzed in both the ground state and the lowest excited triplet state within a density functional theory (DFT) framework. The ultimate goal is to identify macrocycles that deviate from Baird's rule.
The ideal candidate should have a basic understanding of quantum chemistry (commonly acquired in chemistry or physics undergraduate programs) and a strong motivation to learn the fundamentals of electronic structure theory and DFT.
[1] I. Casademont-Reig, E. Matito, and M. Torrent-Sucarrat submitted.
[2] I. Casademont-Reig et al. Phys. Chem. Chem. Phys., 20, 2787 (2018); I. Casademont-Reig et al. Molecules, 25, 711 (2020); I. Casademont-Reig et al. Angew. Chem. Int. Ed., 60, 24080 (2021); I. Casademont-Reig et al. Angew. Chem. Int. Ed., 61, 06836 (2022); I. Casademont-Reig et al. Chem. Eur. J., 29, e202202264 (2023).
