When Aromaticity Changes its Rules
Internship
Type of Project: Theory Project
Location: Donostia
Supervisors: Irene Casademont Reig, Eduard Matito
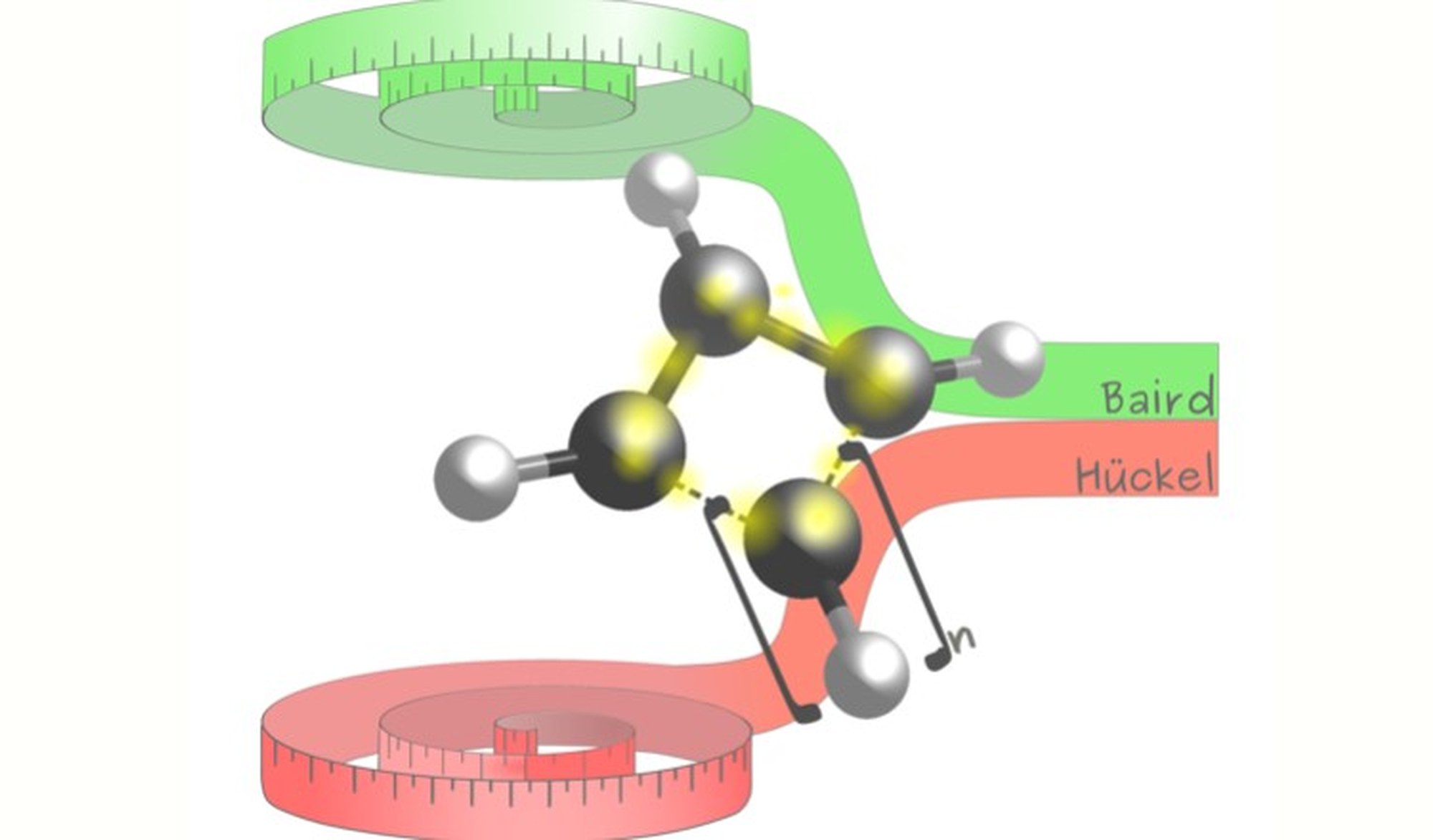
Aromaticity is a cornerstone concept in chemistry that provides a powerful framework to rationalize the structure, stability, and electronic properties of cyclic conjugated molecular systems. Its emergence is commonly rationalized using simple electron-counting rules. In particular, Hückel’s rule predicts that planar π-conjugated systems in their singlet ground state are aromatic when they contain 4n+2 π-electrons, whereas systems with 4n π-electrons are antiaromatic. Remarkably, this picture changes in excited states: according to Baird’s rule, the aromaticity patterns of the ground state are reversed in the lowest triplet state [1-5].
In this two-month internship, the student will investigate aromaticity from a quantitative computational perspective using density functional theory (DFT). Selected molecular systems will be analyzed in both their ground state and lowest triplet excited state using a range of electronic, geometric, and magnetic aromaticity descriptors. A central aspect of the project is the comparison of two complementary computational frameworks: a real-space and a Hilbert-space partitioning schemes, implemented in different quantum-chemistry software packages.
The project provides hands-on training in modern computational chemistry tools, electronic structure calculations, and data analysis. The ideal candidate should have a basic understanding of quantum chemistry (commonly acquired in chemistry or physics undergraduate programs) and a strong motivation to learn the fundamentals of electronic structure theory and DFT. Outstanding candidates might be considered for a PhD position afterwards.
References:
[1] Casademont-Reig I., Woller T., Contreras-García J., Alonso M., Torrent-Sucarrat M., Matito E., Phys. Chem. Chem. Phys. 2018, 20, 2787
[2] Casademont-Reig I., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Molecules 2020, 25, 711
[3] Casademont-Reig I., Guerrero-Avilés R., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Angew. Chem. Int. Ed. 2021, 60, 24080
[4] Casademont-Reig I., Soriano-Agueda L., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Angew. Chem. Int. Ed. 2022, 61, e202206836
[5] Casademont-Reig I., Woller T., García V., Contreras-García J., Tiznado T., Torrent-Sucarrat M., Matito E., Alonso M., Chem. Eur. J. 2023, 29, e202202264
